From the mine to the car

Why Bécancour?
As a global leader in nickel extraction, Vale has embarked on a strategic shift to support the energy transition. Through our partnership with General Motors, are building nickel sulphate plant in Bécancour to supply the Ultium CAM factory. We are excited to join Québec’s emerging battery industry!
Safety
Producing nickel sulphate doesn’t generate significant quantities of greenhouse gasses, and the by-products can be reused, recycled, or eliminated safely. An overview of the project’s impacts is available in our Project notice.
Our process is simple: we place nickel pellets and rounds in a sulphuric acid solution. Once the nickel is dissolved, nickel sulphate is delivered by pipeline to our client, who is on the neighboring lot. The acid is then neutralized leaving only water and salt. Other nickel by-products can be reused.
A thorough environmental assessment
Our project will undergo a thorough environmental assessment by the Québec government. We understand that a fulsome process is essential for our project to obtain the social license to operate. We will ensure the Québec government receives all information it requires as it studies our project.
More infoNickel Sulphate Methodology
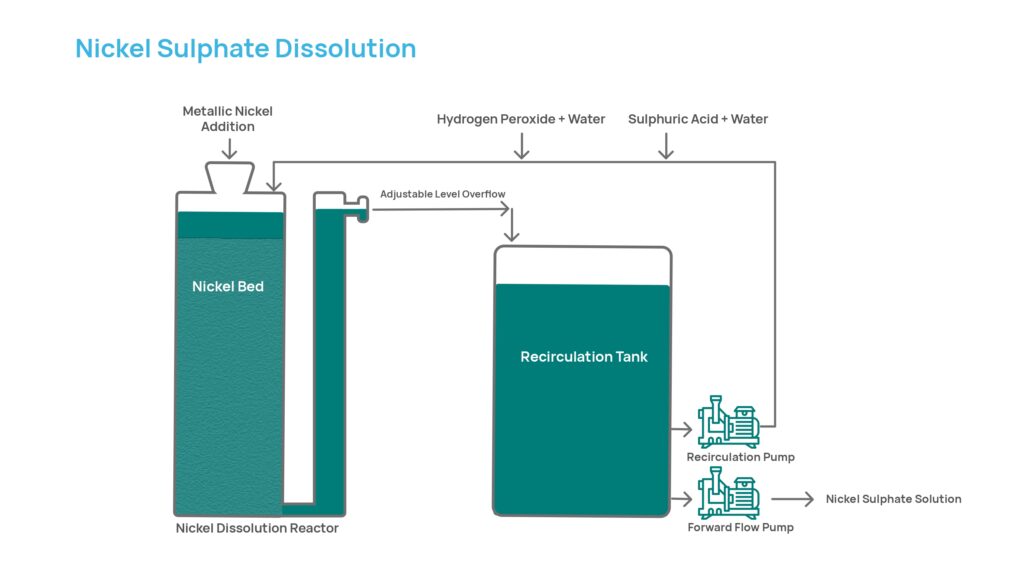
- The process starts with Base Metals high purity nickel metal, in the form of Pellets and Rounds, produced in our refineries in Sudbury and Long Harbour, Canada.
- The Pellets and Rounds are placed into a dissolution reactor and a mixture of sulphuric acid, hydrogen peroxide and clean water is continuously circulated, through the reactor and an external tank, to dissolve the nickel and turn it into a highly pure nickel sulphate solution.
- As the Pellets and Rounds are quite heavy, they form a well packed metal bed in the reactor which is yet sufficiently porous to allow excellent circulation of the dissolving solution.
Nickel Sulphate Dissolution
- The dissolution is carried at moderately elevated temperatures (~60-80°C) fully utilizing the heat generated from the chemical reactions between acid, peroxide and nickel.
- The circulation of the solution through the reactor helps ensure the right temperature for the dissolution process.
- A portion of the pure nickel sulphate solution, along with some residual sulphuric acid (typically 20-30 g/L acid) is continuously withdrawn from the recirculation tank.
- The withdrawn solution is continuously replaced with fresh acid, peroxide, water and more Pellets and Rounds are added through the top of the reactor to replace the nickel being dissolved.
- The nickel sulphate solution is then purified to battery-grade quality for use in lithium EV batteries.
The development of this process was carried out at our corporate technology center in Mississauga, Ontario, leveraging decades of experience with nickel processing innovation, including dissolving metallic nickel to produce high purity nickel sulphate and nickel chloride at our various refineries.